We know that a solution is made up of a solvent and a solute. Nor do we definitely know that we actually do remember negative interactio.

Raoult And Henry Law For Activity And Fugacity
The vapor pressure in the gas phase in such cases is less than that predicted by law which can be explained by the formation of hydrogen bonds between the.
Raoult's law deviation examples. Positive deviation from Raoults law. Department of chemical and biological engineering university of colorado boulder. In pure ethanol molecules are hydrogen bonded.
Raoults Law Example. If the vapour pressure is higher then the solution is said to exhibit positive deviation And if the vapour pressure is lower than the solution then it said to be a negative deviation. First we do not definitively know that the brain is hardwired this way.
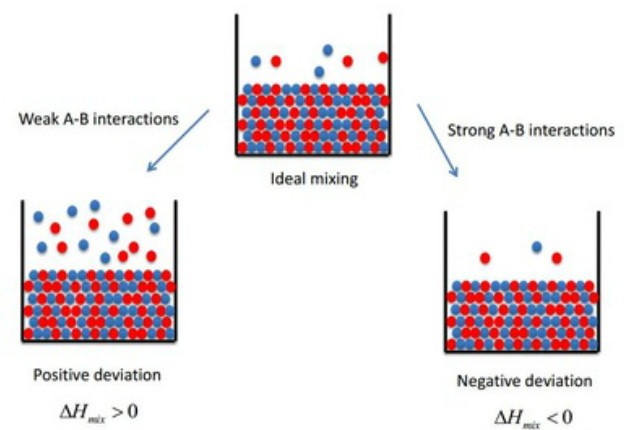
Positive deviation from raoults law occurs when the vapour pressure of the component is greater than what is. The negative deviation occurs when the vapour pressure is lower than expected from Raoults law. Raoults Law Negative Deviation Example.
Raoults Law Deviation Examples. Methyl Alcohol and Water. Acetone and Carbon disulphide.
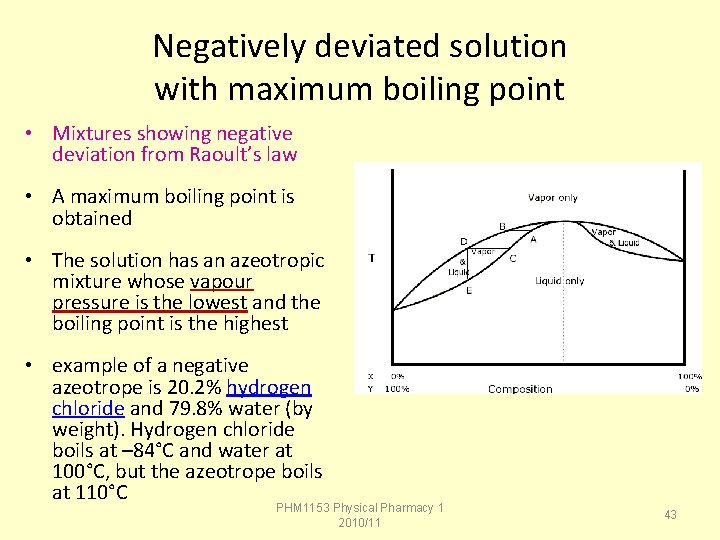
The system shows negative deviation from raoult s law over the entire range of composition the pyridine trichloromethane system3 shown in figure 142b is an example of one with negative deviations from raoult s law. An example of negative deviation is a mixture of chloroform and acetone or a solution of water and hydrochloric acid. Water air while the minor component of a solution is the solute sugar carbon dioxide etc.
For example the mixture of chloroform CH 3 Cl and acetone CH 3 COCH 3 presents a negative deviation from Raoults law. Negative deviation 3Examples 4Graphs 5QuestionsHome work 3. Solution of 68 nitric acid and 32 water by mass.
Following are examples of solutions showing positive deviation from Raoults Law. OH Positive deviation from Raoults law. Carbon Tetrachloride and Toluene or Chloroform.
The non-ideal solutions that do not obey Raoults law over the entire range of concentration and have vapour pressures lower than those predicted by Raoults law show a negative deviation from Raoults law. Ok if you want to remember the negative deviation you can follow this trick. I think it will help you a.
Values of henrys law constants for numerous gasses in different solvents have been measured. Carbon disulphide acetone. For example the system of chloroform chcl 3 and acetone ch 3 coch 3 has a negative deviation from raoults law indicating an attractive interaction between the two components that has been described as a hydrogen bond.
Here H- bonding in methanol is broken by C C l4. A Difference in extent of association in two liquids H2O CH3OH B. Negative Deviation from Raoults Law.
Raoults Law Negative Deviation Example prev question next question Positive deviation from raoults law occurs when the total vapour pressure of the solution is more than corresponding vapour pressure in case of ideal solution. For Example consider two components A and B to form non-ideal solutions. Ideal Nonideal Solutions.
A C C l4. According to Raoults law the partial vapour pressure of each component in any solution is directly proportional to its mole fraction. Raoults Law Types of.
A deviation from raoults law can only be measured experimentally. A mixture of chloroform and acetone In case of solutions showing negative deviations Δ mix H has a. Raoults Law and Examples.
47 Raoults Law Deviation Examples Pictures - Expectare InfoRaoults law plot for a mixture of hexane and heptane. Most of the liquid pairs showing non-ideal behaviour exhibit positive deviations. There is either a negative or a positive deviation.
Positive Deviation from Raoults Law occurs when the vapour pressure of the component is greater than what is expected in Raoults Law. As in very dilute solution the molecular size. A-A interactions are weaker than Either A-A or B-B Tricks to Identify positive or negative deviation in non-deal solutions from Raoults Law Cause of Positive Deviation.
Raoult And Henry Law For Activity And Fugacity. Examples of Positive Deviation. The major component of a solution is the solvent ie.

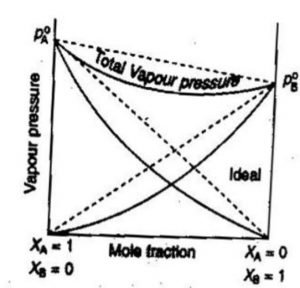
Ideal And Non Ideal Solution Chemistry Class 12 Solutions
Ideal Non Ideal Solutions Raoult S Law Types Of Solutions Videos Q A
Colligative Properties Kausar Ahmad Kulliyyah Of Pharmacy Http
Topic Three Thermodynamics Of Non Ideal Mixtures Chemistry Revision Site

Colligative Properties Kausar Ahmad Kulliyyah Of Pharmacy Http

Deviations From Raoult S Law Youtube

Cbse Class 12 Positive And Negative Deviation From Raoult S Law In Hindi Offered By Unacademy

At What Composition Is Maximum Deviation From Raoult S Law Observed Chemistry Stack Exchange
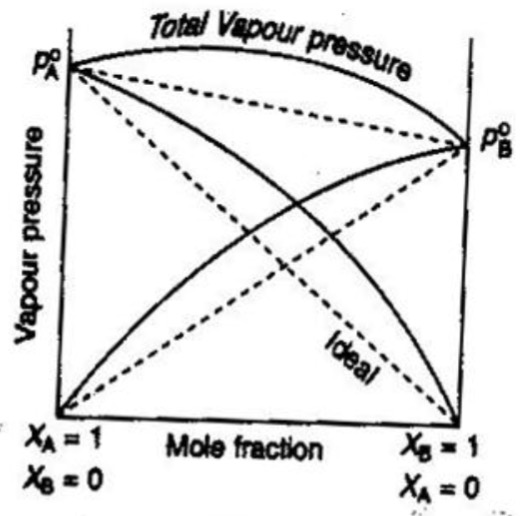
Ideal And Non Ideal Solution Chemistry Class 12 Solutions
Liquid Solutions A Tutorial On Liquid Solutions Notes Figures And Problems With Solutions Target Audience These Notes On Atomic Structure Are Meant For College Freshmen Or High School Students In Grades 11 Or 12 They Might Be Of Use To Indian

1 Chem 212 Chapter 5 Phases And Solutions Dr A Al Saadi Ppt Download
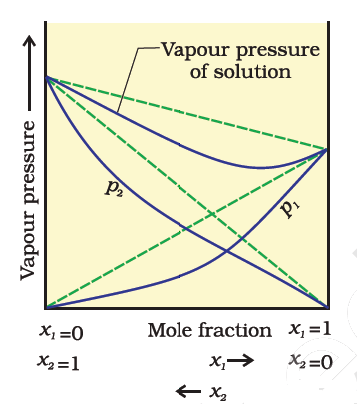
Ideal Solutions And Non Ideal Solutions Their Characteristics

Intro To Solutions We Are Now Going To Use Our Knowledge Of Thermodynamics To Examine Solutions Consider A Solution Of Two Components 1 And 2 The Gibbs Ppt Download

Identify The Mixture That Shows Positive Deviations From Raoult S Law

At What Composition Is Maximum Deviation From Raoult S Law Observed Chemistry Stack Exchange

Ideal Non Ideal Solutions Raoult S Law Types Of Solutions Videos Q A


